Understanding plankton threats to salmon requires a mix of old and new technologies
Using both “traditional” microscopy and environmental DNA (eDNA) analysis can help paint a complete picture of plankton threats to salmon aquaculture, according to University of Glasgow research.1
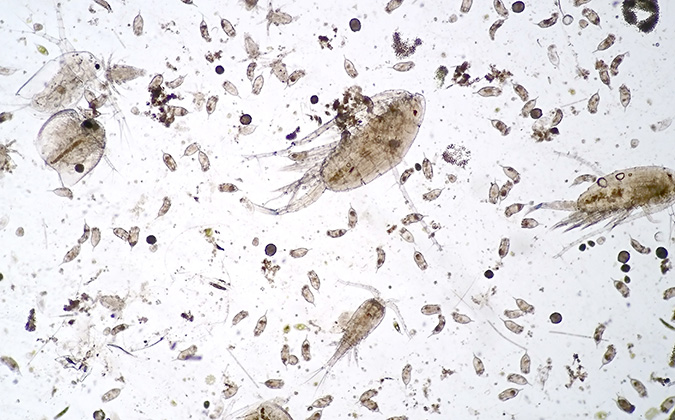
Both zooplankton and phytoplankton have been associated with gill health problems on salmon farms, and the issue is complicated by the effects of climate change on sea conditions. Understanding which species have the biggest impact on fish health is important for the aquaculture industry.
With that in mind, veterinary scientists carried out extensive surveillance at two salmon farms on the west coast of Scotland in the 2021 growing season. They analyzed samples using eDNA techniques, which can identify all species present, as well as more traditional microscopy work.
They found that there was a generally poor correlation between what was found using the different techniques, with some species that pose a threat to salmon health being detected using only one of the approaches. However, there was a stronger relationship between data on phytoplankton derived from the two techniques than zooplankton.
Additionally, the presence of a species’ DNA didn’t shed sufficient light on just how strong a presence they were in the environment — something that was cleared up using microscopy.
All this led the team to conclude that best practice at present would be to use both approaches in tandem, to provide the most comprehensive overview of possible threats to fish stocks.
Spotlight on key planktonic threats
As well as assessing the relative value of the analysis techniques, by using statistical models the team was able to determine links between plankton species and health risks to fish. However, one of the sites studied was sheltered while the other was in the open sea, and this proved to be a crucial factor in determining which species posed the biggest risks.
Despite these differences, there were some high-risk species in common between the sites, namely the genus of single-celled dinoflagellates Ceratium, the larvae of copepods (small crustaceans) and the hydrozoan jellyfish Lizzia blondina.
Reflecting what is generally observed by producers, plankton abundance peaked in the summer months, while the species composition differed between early and late in the season. The scientists also observed delayed effects, where health impacts associated with certain plankton — such as Pseudo-nitzschia, a genus of algae, and Cylindrotheca, a genus of fungi — came after the peak of those species’ presence.
Refining approaches to improve gill health
“For studies that compare traditional and DNA-based approaches, the conclusion that best serves the end goal of surveying biodiversity is often ‘use both’,” the researchers wrote in a paper on their work.
“In some respects, our study is no exception, and no single approach has a clear advantage. However, several potential improvements, especially to the molecular methodologies, could be considered to improve the detection of species of interest.”
Further refinements in reference databases would help, they said, while for some plankton species with known threats to fish, targeted approaches such as quantitative PCR as well as more focused use of DNA sequencing could cut “noise” in the data, such as the inclusion of free-living, non-pathogenic organisms.
“To make generalizations about the extent of the biological threats to salmonid aquaculture, further ‘unbiased’ studies at multiple sites across multiple production cycles are required as well as improving the resolution of the eDNA metabarcoding approach,” they concluded.
“In parallel, to better understand the mechanisms of gill damage and mortality and which organisms drive them, a more detailed monitoring of salmon gill health is required (e.g., gene expression, histopathology) to help disentangle from the role of direct microalgae toxicity and help set abundance threshold for mitigation purposes.”
1 Alguero-Muniz M, Spatharis S, Dwyer T, de Noia M, Cheaib B, Robertson B, Johnstone C, Welsh J, Macphee A, Mazurkiewicz M. High resolution longitudinal molecular and morphological tracking of planktonic threats to salmon aquaculture. bioRxiv. 2023:2023.09.04.556215.






